Around 150 years ago, three scientists named Ernest Rutherford, Hans Geiger, and Ernest Marsden exposed a thin gold foil to radiation. Based on how the rays were deflected by atoms in the foil, they figured out that every atom has a dense centre where its mass and positive charge are concentrated.
Seventy years ago, Robert Hofstadter led a team that bombarded electrons at thin foils. The higher energy of the electrons allowed them to ‘probe’ the nucleus. Based on these interactions, the team understood how charges and magnetic fields were arranged inside a nucleus.
In each case, physicists were able to ‘see’ inside stable atoms, and then inside their nuclei, by using other particles.
Now, researchers in the RIKEN Nishina Center for Accelerator-Based Science, in Japan, have taken a big leap forward in this tradition – by demonstrating a set-up that can use electron scattering to ‘see’ inside unstable nuclei, including those that don’t occur naturally.
The previous experiments used thin foils that were easy to hold. The new one is more sophisticated, using an apparatus to hold the nuclei of caesium-137 atoms as well as make sure electrons could interact with them, using a system called SCRIT. The wait for this advancement is why a similar study couldn’t be conducted before.
The results were published in the journal Physical Review Letters on August 30.
The SCRIT advantage
First, the researchers accelerated electrons in a particle accelerator to energise them, and then smashed them into a block of uranium carbide. This produced a stream of caesium-137 ions (atoms stripped of electrons). This isotope of caesium has a half-life of around 30 years.
“All systems are connected with vacuum pipes and the process is performed in a short time,” Kyo Tsukada, an associate professor at the Institute for Chemical Research, Kyoto University, and the first author of the study’s paper, told The Hindu. “This technique has been developed for short-lived nuclei. If one is only interested in long-lived nuclei, there might be other methods – for example, using the chemical separation of radioactive isotopes.”
The ions were then transported to the SCRIT system, which is short for ‘Self-Confining Radioactive-isotope Ion Target’.
“This method enables us to trap the target ions in three dimensions along the electron beam using the electric attractive force between the ions and the … electrons,” Dr. Tsukada said. The resulting “overlap between the target ions and the electron beam is very good.”
This ‘overlap’ meant that the electrons had a good chance of colliding with the ions. According to Dr. Tsukada, SCRIT allowed the researchers to achieve this with as few as 108 caesium-137 ions. Without SCRIT, they would have required a trillion-times more.
“Furthermore, we recently developed an ion beam generation and beam-stacking system that enables us to extract the caesium-137 unstable nuclei as a pulsed beam immediately after the photo-fission of uranium,” Dr. Tsukada added.
Enter quantum mechanics
The next step was to study the electron-ion interaction.
When light is shined through a tiny, round hole, the shadow on the opposite wall will be concentric circles of light and dark patches. This is because different parts of a light wave passing through the hole are forced to interfere with each other, creating the characteristic interference pattern on the wall.
When an electron is scattered by an atom’s nucleus, it behaves like a wave during the interaction. Once scattered off, the electron-waves interfere with each other. The physicists used a device called a magnetic spectrometer to record the resulting interference pattern. This measurement process has two advantages.
The interactions between particles can become messy. At the Large Hadron Collider in Europe, for example, scientists record vast quantities of proton-proton collision data and analyse them using supercomputers and state-of-the-art algorithms. But interactions involving electrons are much ‘cleaner’ because the theory that describes them is better understood. Information about a nucleus can also be more readily obtained from the electrons’ interference patterns. This is the first advantage.
The second is that the researchers could avoid particle interactions that would invoke more complicated theories by simply fine-tuning the electrons’ energy.
The particles smash
Taken together, the set-up at RIKEN produced some ions, quickly moved them to SCRIT, which readied them for their encounter with the electrons. Then the electrons, which had been accelerated to a particular energy, were smashed into the ions.
Based on the magnetic spectrometer’s readings, the physicists found that the internal structure of a caesium-137 nucleus is consistent with that put together from older studies and theoretical calculations.
The result is important: now, physicists have successfully tested a set-up that can probe the nuclear structure of short-lived atomic nuclei using electron scattering. Caesium-137 is not short-lived but, Dr. Tsukada said, “This experiment is a kind of demonstration of our facility, and caesium-137 was selected as the first example. All procedures are exactly the same as we do for short-lived nuclear targets.”
In other words, the physicists have demonstrated a femtoscope. Just as a light microscope can probe things that are around a micrometre in size, a femtoscope is a machine that can probe the femtometer scale (10-15 m) of atomic nuclei.
This is notable because it’s a new tool that physicists have as they go about tackling an old, unsolved problem: we don’t have a theory today that explains the structure of atomic nuclei.
There are many models that explain the structures of nuclei in different situations, but a common, unified theory that has been verified in experiments remains lacking.
Between the shapes
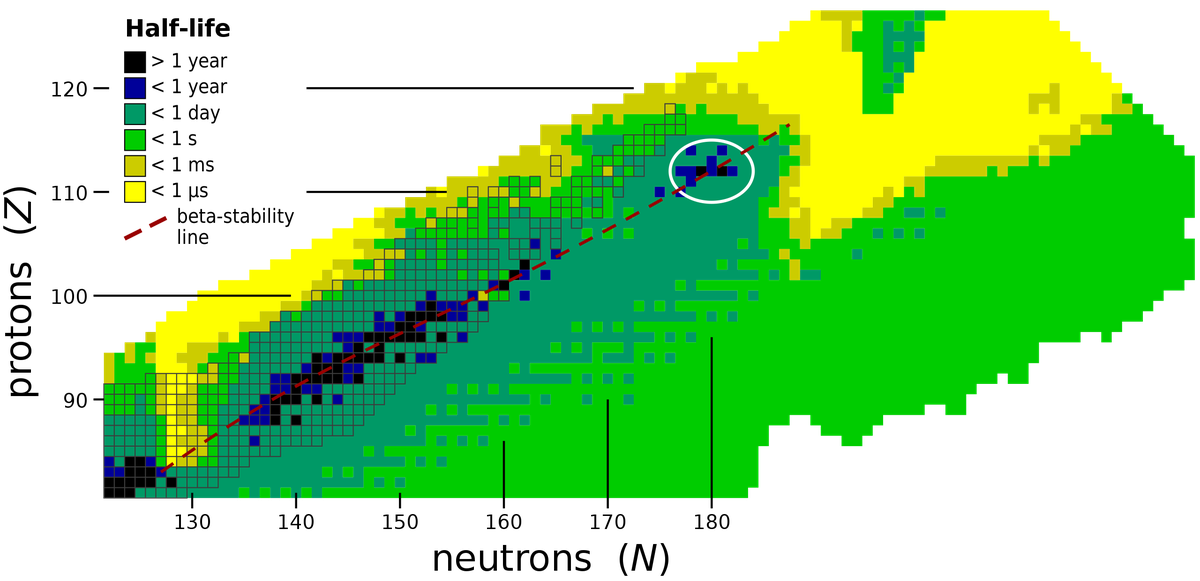
Over the years, physicists have encountered many properties of atoms that emerge from quirks in their nuclei. One example is the ‘island of stability’. Usually, the heavier the nucleus of an unstable element, the faster it will decay via radioactivity. But scientists have found some isotopes that decay slower than their ‘heaviness’ would suggest.
When they plotted a graph with the number of protons on one axis and the number of neutrons on the other, they found that the nuclei of most isotopes lay along a straight line. But they also noticed some isotopes clustered around where the number of protons was 112. This cluster is called the island of stability because these nuclei are unusually more stable. This proton number has become known as the ‘magic number’.
A chart showing the ‘island of stability’ around Z = 112.
| Photo Credit:
Lasunncty (CC BY-SA 4.0)
There are some ideas about how we can explain the existence of the islands – and possibly others like it – but we don’t know for sure. One way physicists hope to fill this gap is by using a femtoscope, like the one the RIKEN team has built, to probe the structures of nuclei that are expected to be oddly shaped.
For example, some unstable nuclei have been hypothesised to have a non-uniform density of protons and neutrons and that they ‘ooze’. With the femtoscope, the hope is that a unifying theory of nuclear structure will be found somewhere in the gaps between expected and unexpected shapes.