Heat is animus. It was there at the birth of the universe, and its death will be the universe’s death. It is impossible to overstate its importance — both throughout human history and across modern technologies. The innovation of steam-powered pumps and engines in the 17th and 18th centuries, reaching the first of many summits in James Watt’s setup in 1764, precipitated the first Industrial Revolution. Today, global warming is forcing us to deliberate on the roles heat plays in our lives.
What is heat?
In the microscope scheme, an object’s temperature is the average kinetic energy of its constituent particles. When two bodies at different temperatures come in contact, the temperature of the cooler one will rise and vice versa; heat here is the amount of thermal energy the bodies have exchanged to effect this temperature change.
Macroscopically, heat is dealt with as a form of energy with specific characteristics, understood using the tools of thermodynamics and statistical mechanics, among other fields.
A medium can absorb heat at one location and dissipate it at another — a possibility that forms the basis of many modern technologies, including thermal and nuclear power plants and air conditioning. Engineers have also developed ways to convert heat into mechanical energy, paving the way for machines like the internal combustion engine.
How is heat used?
Perhaps the best way to understand heat is through an application. Let’s start with two examples: internal combustion engines (ICEs) and thermal power plants.
An ICE converts heat to (mechanical) work, and in this sense is a practical application of a theoretical entity called the Carnot cycle. This cycle describes the maximum thermodynamic efficiency an engine converting heat to work can have. To begin with, the engine has four components: a hot reservoir (a system with more heat), a cold reservoir (a system with less heat), an ideal gas in between (through which heat moves from the hot to the cold reservoirs), and a piston adjacent to the ideal gas. Each cycle has four steps.
In the first step (isothermal expansion), the ideal gas is insulated from the cold reservoir and is exposed to the hot reservoir. Heat moves from the hot reservoir — generated by, say, the combustion of petrol — to the ideal gas. The gas particles are heated up and the gas expands, pushing on the piston. In the second step (isentropic expansion), the gas continues to expand even as it is insulated from both reservoirs, pushing the piston. Its temperature doesn’t change due to the insulation but it loses some energy against the piston. The expansion causes it to cool down as well. In these two steps, the piston has done work on its surroundings.
In the third step (isothermal compression), the gas is exposed to the cold reservoir and deposits its leftover heat there. This time, the piston moves downwards. In the fourth and final step (isentropic compression), the gas is insulated from the reservoirs while the piston continues its downward motion. This act compresses the gas and warms it up again, and the cycle can begin all over again. In the last two steps, the surroundings are said to have done work on the piston.
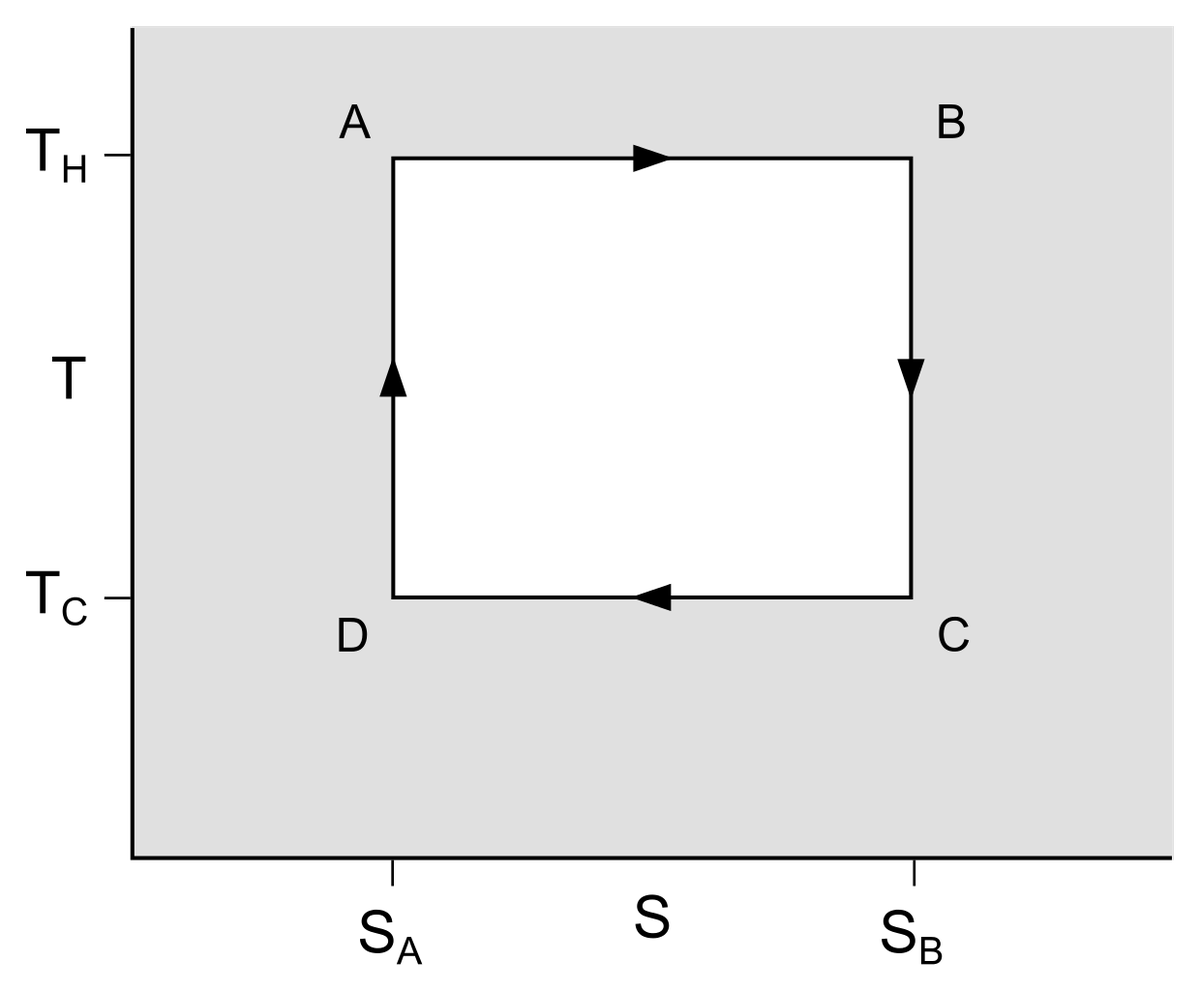
An idealised Carnot cycle visualised on a graph, with entropy (S) on the x axis and temperature on the y axis. T_H and T_C are the temperatures of the cold and the cold reservoirs, respectively.
| Photo Credit:
Public domain
Similarly, in a thermal power plant, the main components are boiler, turbine, generator, condenser, and of course pumps. Let’s assume the working fluid here is water. The ideal form of this system is the Rankine cycle, which also plays out in four steps.
In the first (isentropic compression), a pump compresses the water to a high pressure. In the second (heat addition), the water is pumped to the boiler. Here, the water is heated from an energy source — like burning coal or nuclear fission — while the high pressure is maintained, turning the liquid into a saturated vapour (i.e. just about to condense).
In the third step (isentropic expansion), the pressurised vapour is pumped to the turbine, where it expands to release heat and its pressure drops. The expansion drives the turbine’s blades, which then produce power through a generator. The cooled vapour is pumped to the condenser in the final step (heat removal), where it is condensed at a fixed pressure back to a saturated liquid form (i.e. just about to vaporise). The condenser is functionally a heat exchanger, where a circulating coolant like cold water takes heat away from the vapour.

How is heat related to work?
Heat and work have the same physical dimensions. However, not all types of heat can translate to work. For example, if a system does work while also falling out of thermal equilibrium, it will lose some energy in the process. This can happen in an ICE if, say, the machine isn’t well-lubricated and the piston’s movement against the walls of the combustion chamber experiences friction.
Such loss of ‘useful heat’ is closely related to the concept of entropy, which represents a sort of disorderliness in a system such that the corresponding heat cannot contribute to useful work.
Likewise, when a system does work without losing or gaining heat energy — as in the isentropic expansion and compression steps of the Carnot cycle — the process is said to be adiabatic. Completely adiabatic processes are reversible.
The various components of ICEs and thermal power plants are designed to alternate some medium that is transporting heat between different states, in steps that maximise the amount of work and minimise entropy changes and other energy leaks.
What are some applications of heat?
Understanding the microscopic and macroscopic characteristics of heat has been crucial for metallurgy and materials science, mining, refineries, a large variety of chemical reactions, semiconductor electronics, meteorology, and transportation, among other areas.
Heat also has a starring role in heating, ventilation, and air-conditioning (HVAC) systems. Many cold countries generate and transport heat to homes and offices from centralised facilities, while individual homes also use electric heaters — which convert electric energy to heat energy by passing an electric current through a resistor — to keep people warm. Of late, many experts have articulated a ‘right to air-conditioning’ for the people of low- and middle-income countries suffering debilitating heat.
Heat engines like ICEs and steam engines use the Carnot cycle. Heat pumps, which are air-conditioners that warm the air instead of cooling it, use the reverse Carnot cycle. Similarly, air-conditioners that are used to cool large spaces, like halls, and the insides of cars use the reverse Rankine cycle. Other cycles, depending on the heat-transporting medium and the desired operating conditions, include the Brayton, gas-generator, regenerative, Siemens, and Stirling cycles.

How is heat implicated in climate change?
The world is responding to climate change on two fronts: mitigation and adaptation. Vis-à-vis climate mitigation, researchers around the world are devising new ways to produce heat energy for various applications without involving the combustion of fossil fuels and/or finding ways to reduce emissions from existing technologies — while policymakers are finding new ways to incentivise the uptake of these solutions.
Heat in climate adaptation is most familiar to Indians in the form of heat waves. During a heat wave, how healthy the body already is and how well it can prevent the accumulation of heat are very important. The former depends on long-term living conditions, access to clean living conditions, and access to healthcare; the latter speaks to the short-term means available for a body to slow its accumulation of heat. Beyond a wet-bulb temperature of around 32 degrees C, even short durations of non-strenuous outdoor activity can cause grievous harm.
Of course, global warming itself is fundamentally a heat problem. Of the energy coming to the earth from the Sun, some is reflected, some is absorbed by the atmosphere, and some warms the ground. At night-time, the planet releases this absorbed energy in the form of infrared radiation. Greenhouse gases, including carbon dioxide, methane, and water vapour, absorb this radiation, translate it to kinetic energy and warm up the atmosphere, reducing the efficiency with which the earth’s surface can cool down.