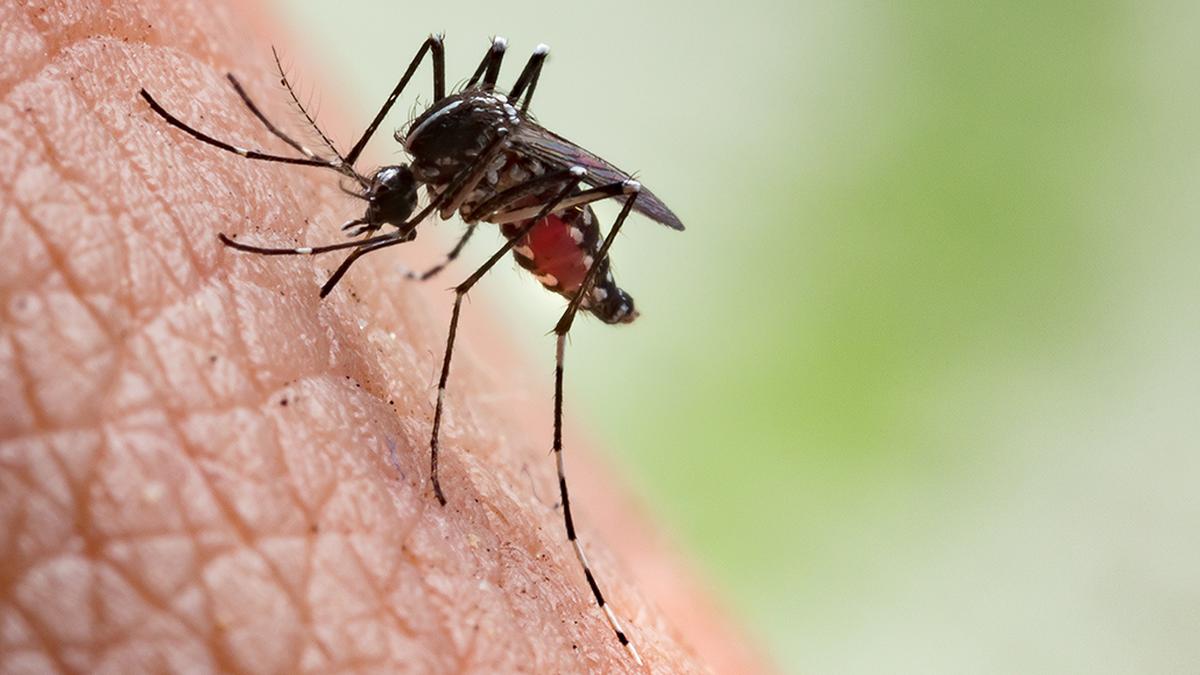
A recent case of Zika virus infection in Pune has renewed concerns about India’s preparedness for diagnosing emerging infectious diseases. After experiencing symptoms like fever and rashes, the 46-year-old doctor was hospitalised and diagnosed with Zika virus infection after his samples were sent for testing to the apex virology institute. Subsequent testing of his family members revealed that his 15-year-old daughter was also infected. This is not the first time Zika has been identified in India. Cases have been identified from multiple States in India in the past, with larger outbreaks occurring in Kerala and Uttar Pradesh as recently as 2021.
Zika virus is a mosquito-borne pathogen belonging to the flavivirus family of viruses which also includes dengue. Clinical symptoms of Zika infection in many cases could be mild and indistinguishable from other infectious diseases including dengue. However, for pregnant women, the Zika virus poses a significant risk as it can be transmitted from mother to child, potentially leading to microcephaly in the offspring.
Due to climate change, it is not surprising that multiple dengue outbreaks are making headlines. The same vectors that spread dengue could also spread Zika. However, India’s lack of significant Zika surveillance means we might never fully understand its spread. In March 2023, CDSCO, India’s apex organisation for diagnostic approvals, confirmed that there is no approved diagnostic test for Zika. This limitation hinders our ability to diagnose Zika, relying only on classical symptoms and high clinical suspicion, making it complex as we see a concurrent upsurge in dengue cases across the country. Surveillance by ICMR on Aedes mosquitoes showed Zika virus positivity following human cases, indicating that many cases are likely being missed.
Zika is not an isolated case. A case of avian influenza A/H5N1 was recently reported from Australia in a child who had traveled to India, hinting at more undetected infections. Despite multiple avian influenza outbreaks in India this year affecting poultry, and an ongoing outbreak in Kerala, human testing and surveillance have remained limited. This is partially compounded by the lack of widely available diagnostic tests and over-reliance on a few apex institutes.
Consider the case of the Nipah virus, which has seen multiple outbreaks in Kerala. India has experienced several Nipah virus outbreaks, notably in West Bengal (2001 and 2007) and Kerala (2018, 2021, and 2023). In Kerala, identification of the virus relied heavily on clinical suspicion. Some cases were indeed missed during initial admissions due to the lack of routine testing, largely because the diagnostic facilities are not readily available apart from apex national institutes, adding to the complexity of testing, delays, and consequent loss of valuable time to institute countermeasures. Rapid identification and isolation of cases, contact tracing, and targeted screening of contacts are key to the effective containment of Nipah outbreaks.
For the rapid development of widely available and accessible diagnostics, the primary requirement is the quick availability of whole genome sequences in the public domain from outbreaks apart from an independent, time-bound accreditation process, especially in outbreaks. While many countries grant emergency approvals for diagnostics based on synthetic genomic material, India requires validation on clinical samples, which are not readily accessible. These limitations hamper rapid development. Although there have been multiple publications on Zika and Nipah outbreaks over many years now, the genomes from these outbreaks are still not rapidly released in public repositories. For example, the Nipah virus genome from the 2023 outbreak in Kerala was only released last month. Even with ongoing avian influenza outbreaks in multiple States, we do not yet have the whole genome sequences available in GISAID, the primary repository for influenza sequences, hampering our understanding of the disease and spread and more importantly, our ability to rapidly develop and deploy diagnostics.
During the COVID-19 pandemic, India swiftly expanded its testing infrastructure by rapidly decentralising and leveraging the existing network of hospitals, medical colleges, and private laboratories nationwide and tapping into the industry with a systematic approach for approvals of diagnostic tests making diagnostics widely available and accessible. The experience gained from the COVID-19 pandemic can be a stepping stone for improving testing capacities for other emerging diseases.
By decentralising testing facilities particularly at the district and sub-district levels, developing accessible and affordable diagnostic tests for Zika, Nipah, avian influenza, and many more emerging infectious diseases, India can ensure a more effective response to future outbreaks. There has never been a better time to swiftly establish a decentralised system for diagnostics, genomic surveillance, and data sharing to enable preparedness and public health response to emerging infectious diseases.
(Bani Jolly is a senior scientist at Karkinos Healthcare, and Vinod Scaria is a senior consultant at Vishwanath Cancer Care Foundation and Adjunct Professor at IIT Kanpur and DY Patil Vidyapeeth)