Victor Ambros and Gary Ruvkun won the 2024 Nobel Prize in Physiology or Medicine for discovering microRNAs and their role in controlling gene expression. This pioneering discovery was made using the roundworm Caenorhabditis elegans. This 1-mm long, slender, and transparent nematode has been the star of many pathbreaking discoveries in biology, four of which have won Nobel Prizes.
How did C. elegans, a tiny invertebrate, become such a crucial tool for biological research? What insights did this worm yield? What is the value of such research for society when one can argue that our focus should be on studying human biology?
This is the story of C. elegans. In brief, advances necessary for human health and welfare often arise from solving fundamental biological problems. One major difficulty isn’t just finding the right question to ask but also finding the right place to ask it where it can be solved. C. elegans provides exactly such a setting: a relatively simple but versatile model for biological investigations whose results often reveal general principles that remain valid or have parallels in other organisms, including humans. The worm’s story also highlights how breakthroughs can arise from research driven by curiosity.
Humble beginnings
In 1963, biologist Sydney Brenner wrote to his peer Max Perutz his thoughts on research in the fields of development and neurobiology. He believed that, as the nature of problems in these areas wasn’t clearly defined, there was a gap in identifying the right experimental approach that would lead to “defining unitary steps of any given process”.
Brenner suggested the use of genetic analysis in defining such unitary steps in both animal development and the nervous system. He also indicated his choice of using a metazoan organism, which was likely to be the roundworm Caenorhabditis briggsae. But Brenner later chose the nematode C. elegans, a close relative of C. briggsae, for a number of reasons such as its small size, short life cycle (~3.5 days), small genome, transparent body, and its small number of 302 neurons — unlike the billions of neurons in the human brain. Additionally, C. elegans has several organ systems akin to those found in humans, allowing a chance to identify principles in development.
Ellsworth Dougherty was among the early scientists to realise and use the potential of free-living nematodes in genetics research. In 1963, Brenner requested Dougherty for a culture of C. elegans and also sought his guidance on its growth conditions. This collaboration sowed the seeds for building a community that shared resources and collectively contributed to advancing biology research. This culture of sharing resources and unpublished information continues to this day.
Cell death
Brenner shared the 2002 Nobel Prize for medicine with H. Robert Horvitz and John Sulston “for their discoveries concerning genetic regulation of organ development and programmed cell death”. In his award ceremony, Brenner said, “Without doubt the fourth winner of the Nobel Prize this year is Caenorhabditis elegans: it deserves all of the honour but, of course, it will not be able to share the monetary award”.
Brenner established C. elegans as a genetic model and demonstrated that genes in the wormcould be mutated, resulting in observable changes in development and behaviour.
Extending Brenner’s work, Sulston in 1976 elucidated the cell lineage of C. elegans, which is the developmental history of all cells of this nematode. He tracked cell divisions from the fertilised single cell to the final 959 cells in the adult organism. This was possible because of the transparency of the worm, but nonetheless was a daunting task.
While mapping the lineage, Sulston observed that some cells of a lineage died at specific time points during the normal development of a healthy worm. He showed that precisely 131 of the 1,090 cells born died during development and that cell death was genetically controlled. He described the steps involved in programmed cell death, where healthy cells killed themselves. He also identified a gene responsible for degrading the DNA of the dying cells.
The genes required for the initial steps of programmed cell death were still unknown. Horvitz continued to identify genes essential for cell death and genes that prevented cell death through mutant generation followed by genetic analyses. He found that the process of cell death arose from interactions among key genes and followed a specific molecular pathway.
His team’s work in C. elegans showed that many genes involved in cell death also have counterparts in humans. Research in C. elegans was particularly important in advancing understanding of the role of programmed cell death in human development, e.g. of fingers and the nervous system as well as in some cancers.
Ageing and genome sequencing
Work in C. elegans also led to an understanding of the pathways that regulate ageing. The early work from Michael Klass, Tom Johnson, and Cynthia Kenyon in the 1980s and 1990s identified some of the genes leading to longer lifespan than seen in normal worms. Further work in this direction led to the appreciation of the role of insulin signaling pathways in ageing. The insulin signaling pathway plays the same role in ageing in flies and mice and is associated with longevity in humans. This has led to C. elegans being used as a key model for discovering the molecular mechanisms of ageing and as a test bed for drugs that might influence this process.
The genome information of any organism is invaluable in linking phenotypes, i.e. observable characteristics, to a particular gene. Sequencing the C. elegans genome started in 1990 and was an exemplar for the larger Human Genome Project. The whole genome sequence of C. elegans was carried out by a consortium working together across continents. It was led by Robert Waterston at the Genome Sequencing Center at Washington University in the USA and Richard Durbin at the Sanger Centre in the UK.
The technology and the software tools developed for sequencing the C. elegans genome led the way in achieving the scale and efficiency critical for sequencing of larger genomes. It was debated if human whole-genome sequencing data should be publicly available as private sequencing efforts wished to patent some of the genes. The open sharing of C. elegans data and community feedback on annotation provided a model for the public human genome sequencing effort.
Gene silencing
A geneticist’s dream is to control gene expression, which is the ability to turn genes ‘on’ or ‘off’ in a controlled manner. The traditional way to begin by finding suitable mutants is often time-consuming.
In a landmark 1998 paper, Andrew Fire and Craig Mello discovered RNA interference, several of its salient features, and its potential as a major research tool, one that biologists now use every day. Fire and Mello found that in C. elegans, double-stranded RNA (dsRNA) was more effective at causing uncoordinated movement of the worm than single-stranded RNA. Injecting a small amount of dsRNA could lead to destruction of a much larger amount of the corresponding cellular RNA, suggesting dsRNA was a catalyst for RNA interference.
For their work, Fire and Mello jointly received the 2006 Nobel Prize for medicine.
The most common application of RNAi is to reveal genes’ functions. This technology led to efforts for whole genome RNAi in C. elegans, more defined efforts against gene subsets in other organisms, and has become a staple in the biologist’s toolkit to study gene function. The discovery of RNAi also led to technologies for a highly specific approach to gene-silencing that work in all known organisms. They also have applications in therapeutics for cancer and some inherited gene disorders.
Glowing worms
Another dream of biologists is to track biological processes and gene products in living cells. The green fluorescent protein (GFP) has revolutionised our ability to do this. How this came about is another success story of curiosity-based research.
It started with Osamu Shimomura, who was trying to understand why jellyfish are fluorescent. He identified bioluminescent proteins like GFP from the jellyfish Aequorea victoria in the 1960s. In the late 1980s, Martin Chalfie learnt of Shimomura’s discovery about jellyfish while listening to a seminar by another scientist and immediately wanted to try using GFP to locate where some important genes were expressed.
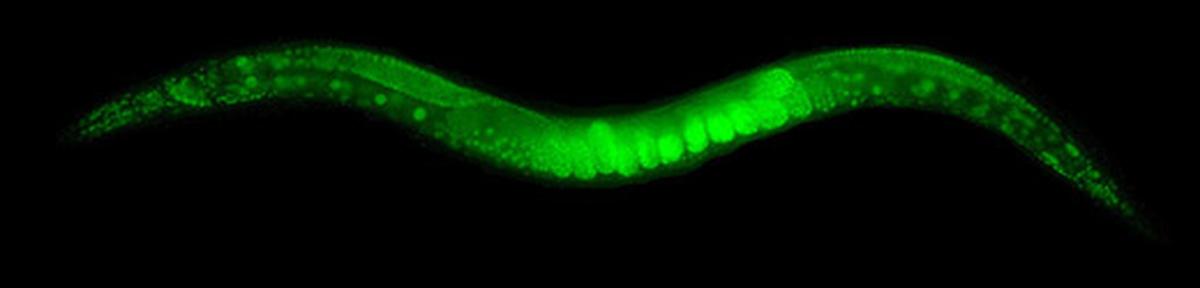
Until then, this effort was labour-intensive and required the organism to be killed. In 1994, Chalfie was able to introduce GFP into live C. elegans. The transparency of the organism meant easy visualisation: he saw green glowing cells when the organism was illuminated by blue light.
GFP was soon widely used in many organisms and cells and has transformed biological research. Together with Roger Tsien’s success in making a ‘rainbow’ of fluorescent proteins of multiple colours, today scientists can follow multiple processes and proteins simultaneously in a wide variety of organisms.
Shimomura, Chalfie, and Tsien received the 2008 Nobel Prize in chemistry for developing GFP.
An adult C. elegans worm glows after a GFP coding sequence was inserted into it.
| Photo Credit:
Dan Dickinson, Goldstein lab, UNC Chapel Hill (CC BY-SA 3.0)
The microRNA prize
The discovery of microRNAs (miRNA) challenged the central dogma, which said RNAs were mere conduits of information, from DNA to proteins. We now know that miRNAs are a class of regulatory molecules that turn off gene expression at the right time and place. This fundamental advance, which won a Nobel Prize in 2024, is another example of an unexpected finding arising from curiosity.
The story starts with the study of lin-4 and lin-14, two genes with opposing effects on developmental timing in C. elegans. In the 1990s, Victor Ambros was studying the molecular nature of lin-4 and Gary Ruvkun, that of lin-14. Ambros found that lin-4 makes a tiny RNA (later called miRNA) which is itself important for gene function and which doesn’t make protein at all. He and Ruvkun together realised that this miRNA could bind to lin-14.
Ruvkun went on to show that the lin-4 miRNA regulates lin-14 by reducing the latter’s protein expression, providing the first demonstration of a regulatory role for a miRNA. Ruvkun also identified let-7, another miRNA that controls developmental timing and is evolutionarily conserved across the animal kingdom.
The sequenced C. elegans genome, RNAi, and computational approaches together led to the identification of several other miRNA genes. It’s established that gene regulation by miRNAs is an essential process during development and for normal physiological processes of organisms. RNAi and miRNAs use related pathways to make small RNA fragments that control gene expression. As work in this area has exploded, miRNAs have also been shown to activate genes and move between cells. With the expanding role of miRNAs there is recognition of potential applications in diagnostics and therapeutics.
There are interesting human aspects to this story, including a connection with Indian science. Ambros and Ruvkun both began their work as postdoctoral fellows in the laboratory of Horvitz, the 2002 laureate, and continued to discuss their work when they had their own independent laboratories. They discovered the direct connection of lin-4 miRNA with lin-14 in a late night phone call, when they both read out the gene sequences of lin-4 and lin-14 to each other.
The mutant gene lin-4 was isolated in the lab of fellow 2002 laureate Sydney Brenner. The variant that Ambros pursued (e912) was isolated by P. Babu, a faculty member at the Tata Institute of Fundamental Research, Mumbai, who was visiting Brenner. Babu went on to set up the first C. elegans lab in India at the institute.
A prize for neuronal circuits
Even though C. elegans has just 302 neurons, it still exhibits complex behaviour. Thus it offers a promising model to study a nervous system that is simple enough to analyse while still yielding valuable lessons about general principles.
Naturally, a very useful step is to look for a layout of all its neurons. Brenner began such a reconstruction of the C. elegans nervous system with all its connections in the 1970s. It was a formidable challenge, never attempted before. John White, Brenner’s PhD student, set up a computer system for neuron reconstructions from electron micrographs. He stayed on after his PhD to reconstruct the C. elegans connectome — a map of all neuronal connections — and published it in a 1984 monograph, ‘The Mind of the Worm’.
This was done well before modern technological tools were available and was the first such effort for any organism. To highlight the scale of difficulties: only in the last two years have we built the connectome of the fruit fly (Drosophila melanogaster) and of a 1 cubic mm chunk of the human brain. The connectome immediately opened the door to questions about how neural circuits function. For example, in the 1980s, Chalfie (the 2008 laureate for GFP) used laser-based neuron-killing experiments to determine the circuit for the escape response to touch.
We can ask questions more broadly about the relationship between the genetics of neural circuits and behaviour. The answers, when we can find them in specific contexts, are difficult to obtain, extremely complex, and yet vital for our understanding. Cornelia Bargmann won the 2013 Breakthrough Prize in Life Sciences for her research on the olfactory system of C. elegans. The sense of smell is very important for many organisms. C. elegans has a large number of olfactory receptors and uses complex machinery to sense smells, and respond, adapt, and learn.
Bargmann conducted a determined assault on this complex problem, achieving remarkable insights in all these aspects by conducting elegant experiments. As a postdoctoral fellow with Horvitz, she identified smell neurons in C. elegans. In her own laboratory, she took a molecular genetic approach and identified proteins involved in sensing odours. Her lab also showed that despite there being a fixed template for sensing attractive and repulsive cues, there was great flexibility in the olfactory circuit, achieved via individual genetic variation as well as the ability to integrate environmental cues.
Several of these findings hold true in organisms like mice, with promise for understanding the human brain.
The worm’s story
C. elegans research was launched by Brenner in the intellectually fertile and exciting environment at the Laboratory of Molecular Biology at the University of Cambridge. In its early years the programme attracted Chalfie, Fire, Horvitz, Kenyon, Sulston, Waterston, White, and other talented young people. These early researchers and those they trained made major contributions to worm research and, thanks to the universality of biology across organisms, fueled major scientific discoveries. These researchers also built a culture of curiosity-driven science and a community with open scientific exchange.
There are many thrust areas where C. elegans research continues to make contributions, such as network behaviour of the nervous system, ageing, and the innate immune response. While it is hard to predict where the next major scientific breakthroughs will arise, the C. elegans story illustrates how curiosity can lead to them, with potentially significant lessons for our understanding of nature and benefits for humankind.
Rohini Karandikar is a science communicator, educator, and facilitator. Sandhya Koushika is a cellular neurobiologist who works with C. elegans at the Tata Institute of Fundamental Research, Mumbai.
Published – January 08, 2025 05:30 am IST